-
Lanthanide-Mediated Supramolecular Cages and Host–Guest Interactions
B. El Aroussi, L. Guénée, P. Pal and J. Hamacek
Inorganic Chemistry, 50 (17) (2011), p8588-8597


DOI:10.1021/ic201156q | unige:17235 | Abstract | Article HTML | Article PDF
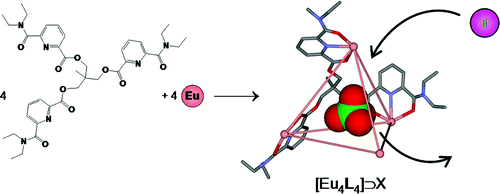
The structure and thermodynamic properties of lanthanide complexes with a new tripodal ligand L2 have been elucidated using different physicochemical methods. At stoichiometric ratios, the tetrahedral three-dimensional complexes with lanthanide cations are formed in acetonitrile with good stabilities. Despite minor structural changes comparing to previously investigated tripodal ligands, the resulting assembly exhibits different features revealed with the crystal structure of [Eu4L24](OH)(ClO4)11 (orthorhombic, Pbcn). Interestingly, the highly charged edifice contains an inner cage encapsulating a perchlorate anion. Such lanthanide mediated cage-like assemblies are rare, and may be of interest for different sensing applications. Indeed, the anionic guest can be exchanged with different anions. The related host–guest equilibria were investigated with NMR techniques. Various aspects of these reactions are qualitatively discussed.

